Abstract
Introduction: The source of chimeric antigen receptor (CAR) T cells can be autologous or allogeneic, depending on the history of allogeneic hematopoietic stem cell transplantation (allo-HSCT). For patients with a history of allo-HSCT, CAR T cells are allogeneic, which can be manufactured from donors' peripheral blood mononuclear cells (PBMC) (donor-derived allogeneic CAR T cells, DD-alloCAR) or patients' own PBMC (recipient-derived allogeneic CAR T cells, RD-alloCAR). Unlike autoCAR T cells, which are activated by CAR only, alloCAR T cells receive activation signals from both T-cell receptor and CAR. The difference could affect CAR T-cell biology, resulting in different clinical outcomes.
Methods: We retrospectively reviewed 31 consecutive patients who received CAR T-cell therapy in our center. Patients were divided into three groups, as autoCAR group, DD-alloCAR group and RD-alloCAR group. We compared the efficacy and safety profiles among the three groups. We also performed a subgroup analysis of patients received alloCAR.
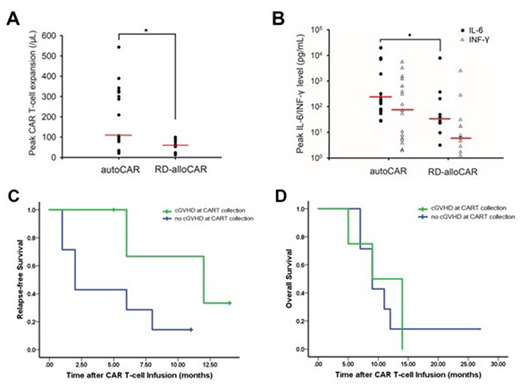
Results: Altogether there were 17 patients in the autoCAR, 11 in the RD-alloCAR and 3 in the DD-alloCAR groups. After a median follow-up of 9 months, the CR rate was 88.2% (95% CI 63.6-98.5%) in the autoCAR group and 100% (95% CI 71.5-100%) in the RD-alloCAR group, which had no significant difference (p=0.73). CR rate in DD-alloCAR was 66.7% (95% CI 9.43-99.1%). The median peak expansion of CAR T cells in the autoCAR was significantly higher than the RD-alloCAR group (p=0.007, Figure A). However, the median OS and EFS did not differ significantly among the groups. As far as adverse effects, RD-alloCAR group had significantly less patients with severe CRS (defined as Grade ≥ 3) than the autoCAR group (p=0.049), with significantly lower peak IL-6 level (p=0.021, Figure B). Neurotoxicity and the degree of cytopenia did not have significant difference among the groups. Acute GVHD occurred in 2 (18.2%) RD-alloCAR patients and 1 (33.3%) DD-alloCAR patients following CAR T-cell infusion. Univariate subgroup analysis of the patients who received alloCAR T-cell therapy showed the presence of cGVHD at the time of PBMC collection was significantly associated with less 6-month relapses (p=0.022); the median RFS in the no-cGVHD group was 2.00 (95% CI 0.72 to 3.28) months, compared to 12.0 (95% CI 2.40 to 21.6) months in the cGVHD group (p=0.084, Figure C); the median OS had no significant difference (Figure D). RD-alloCAR patients with or without cGVHD at PBMC collection did not differ in terms of the peak CAR T-cell expansion, CRS grades or OS.
Conclusions: Compared with autoCAR, RD-alloCAR had similar efficacy but less severe CRS. For RD-alloCAR T-cell therapy, the presence of cGVHD at the time of PBMC collection was associated with fewer 6-month relapses. DD-alloCAR and RD-alloCAR T-cell therapies were effective and safe without causing significant GVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal